Upcoming Webinars
 In this webinar we will critically review the current methods for generating early developability data, identify existing shortcomings and present innovative high-throughput assays utilizing the plate-based PAIA technology. Attendees will learn about how to use this method which is designed to deliver reliable and unambiguous biophysical developability data, serving as an efficient replacement for time-intensive and laborious HPLC and ELISA methods.
In this webinar we will critically review the current methods for generating early developability data, identify existing shortcomings and present innovative high-throughput assays utilizing the plate-based PAIA technology. Attendees will learn about how to use this method which is designed to deliver reliable and unambiguous biophysical developability data, serving as an efficient replacement for time-intensive and laborious HPLC and ELISA methods.
Key Takeaways:
- Review of current methods for early developability data generation.
- Identification of shortcomings in existing techniques.
- Introduction to PAIA high-throughput assays for efficient biophysical data collection.
About Speaker:
Sebastian Giehring earned his PhD in analytical chemistry from the University of Hamburg. He subsequently worked in a scientific role at a spectrometry company before transitioning to a venture capital firm as an investment manager for life sciences. In 2006, he chose to change to the startup sector, taking on various management roles in life science tool companies. In 2014, he invented the PAIA microplate technology and founded PAIA Biotech GmbH, where he has served as CEO ever since.
Register here to secure your spot!
On Demand Webinars
Selected webinars can also be found on our YouTube channel
 Bispecific antibodies (bsAbs) are making waves in the biotherapeutics world, especially in oncology and immunotherapy. But how can we accelerate the discovery process and pinpoint the best candidates? In this webinar, learn how Bio-Rad Laboratories SpyLock Technology is transforming bispecific antibody discovery!
Bispecific antibodies (bsAbs) are making waves in the biotherapeutics world, especially in oncology and immunotherapy. But how can we accelerate the discovery process and pinpoint the best candidates? In this webinar, learn how Bio-Rad Laboratories SpyLock Technology is transforming bispecific antibody discovery!
In this webinar, Dr. Francisco Ylera, head of R&D at Bio-Rad Laboratories, will share how SpyLock Technology is revolutionizing bispecific antibody discovery!
You will learn:
• How SpyLock uses the SpyCatcher-SpyTag system to quickly assemble and screen hundreds of bsAbs
• Integration with Bio-Rad’s Pioneer™ Antibody Discovery Platform for high-throughput screening
• A real-world case study showcasing how SpyLock has helped identify the most promising bispecific candidates
This is a great opportunity for anyone looking to stay ahead in bispecific antibody research!
About speaker:
Francisco Ylera is the head of the R&D team that generated the Pioneer Antibody Discovery Platform and developed the SpyLock Technology for bispecific generation and screening. He has been with Bio-Rad for over 20 years and works on customer antibody generation projects designing the selection, antibody characterization, and method optimizations. Francisco has contributed to over 25 publications, patents and book chapters, earning more than 480 citations. Francisco holds a diploma in chemistry from RWTH Aachen University, Germany, and a Ph.D. in biochemistry from the Free University Berlin, Germany. After his Ph.D., he did a postdoc at the Harvard Institute of Medicine, Boston, USA.
View the On Demand recording.
Immunogenicity is a critical risk factor in the development of biologics, including monoclonal antibodies, ADCs, recombinant proteins, and cell & gene therapies. It can endanger patient safety and reduce treatment effectiveness.
This webinar will explore how the cutting-edge MAPPs (MHC-Associated Peptide Proteomics) assay, a high-sensitivity in vitro immunopeptidomics approach, can help identify regions within therapeutic proteins prone to HLA binding. By assessing sequence-based risks of activating T cell responses, the MAPPs assay plays a key role in preclinical immunogenicity risk assessments.
Speaker: Dr. Noel Smith, Head of Immunology at Lonza | noel.smith@lonza.com
Dr. Noel Smith earned his PhD at the University of Cambridge in metabolic disease and has been with Lonza since 2009. He has been instrumental in developing assays for immunogenicity and immunotoxicity risk screening and currently leads the Immunology group at Lonza.
Don’t miss the opportunity to deepen your understanding of immunogenicity risk in biologics development.
View the On Demand recording.
Monoclonal antibody discovery has been a foundational innovation that continues to drive novel antibody therapeutics in research areas including cancer, autoimmune disease, and infectious disease. As the demands and requirements for new antibodies continue to evolve, especially around safety, efficacy, and functional characterization, more powerful screening technologies are critical to antibody discovery and therapeutic development success. Unlike hybridoma and display technologies that can limit antibody diversity, have low campaign success, and require months to perform, the Beacon® optofluidic technology enables rapid selection of lead candidates by serial function-first screening of single B cells to increase success rates and shorten antibody discovery timelines to days.
This presentation will first highlight Bruker Cellular Analysis’ full Beacon antibody discovery suite for screening primary plasma cells and memory B cells, including the newest ability to discover antibody hits based on relative binding affinity, and share how customers can leverage optofluidic technology to rapidly discover high value monoclonal antibodies with the highest chances of success and fastest turnaround times.
The second half of this presentation will highlight how GenScript uses the Beacon optofluidic system for initial single B cell screening, followed by the state-of-the-art ProSpeed expression system for rapid re-expression and enabling world-class antibody characterization. Genscript’s ProSpeed™ single B cell screening service transforms lead characterization, including deep functional antibody profiling and enhanced BCR sequence recovery, making it faster and more cost effective than traditional plasmid-based expression systems.
About the speakers:
 Vincent Pai, Director, Product Management, Bruker Cellular Analysis
Vincent Pai, Director, Product Management, Bruker Cellular Analysis
Vincent Pai is Director of Product Management at Bruker Cellular Analysis. He has over 10 years of experience in cell biology, nanotechnology, and microfluidics, with a background from UC San Diego and Massachusetts General Hospital in Boston. He now leads new product development for antibody discovery applications on the Beacon® optofluidic system.
 Yu Liang, PhD, VP of Ab Drug Discovery, GenScript ProBio
Yu Liang, PhD, VP of Ab Drug Discovery, GenScript ProBio
Dr. Yu Liang received his Ph.D. degree in Virology/Microbiology from the University of Chicago and completed his postdoctoral training at NIAID, NIH. He then joined Novartis Inst. for Biomedical Research as a PI/lab head, and WuXi Biologics Discovery as Portfolio Director and Senior Project Leader. Currently he serves as VP of Ab Drug Discovery at GenScript ProBio, a global CDMO company providing end-to-end service from discovery to commercialization in cell and gene therapy (CGT), vaccine and antibody protein drug, to accelerate drug development for global customers. Dr. Liang has over 20 years of research experience in biomedical science in both academia and industry. His broad and in-depth knowledge & experience covers cellular / molecular biology, infectious diseases and molecular virology, viral/ tumor immunology and immunotherapy, and early phase drug discovery of bio-therapeutics, with special expertise in the discovery of therapeutic modalities based on therapeutic Abs (mAbs, BsAbs, ADCs, etc.).
View the On Demand recording
Avoid Thy Neighbor: Antibody Discovery Lessons from Successful LILRB1/LILRB2 Targeted Myeloid Suppression
Dr. Kalyani Mondal, Associate Director BioSensors, Charles River Antibody Discovery
Broadcast date: October 17, 2024
Inhibition of the innate immune system to disrupt “Don’t Eat Me” signals between tumor and macrophages is a rapidly growing area of drug development. LILRB1 and LILRB2 are two such immunomodulatory receptors but targeting them poses the challenges of avoiding ten highly homologous LILR family members that are also expressed on myeloid cells. This presentation provides a rational and successful approach for overcoming these challenges utilizing a fully human, highly diverse antibody phage display library. It will also cover the next stages of antibody discovery and development, reviewing the in vitro and in vivo characterization required to go from antibody discovery to clinical candidate.
View the On Demand recording
View the webinar slides
Accelerating Antibody Drug Discovery with Fully Human Antibody Mouse HUGO-Ab and High-Throughput Single B Cell Screening
Dr. Shun Zhou, R&D Director at Cyagen Bioscience
Broadcast date: July 25, 2024
In this webinar, Dr. Shun Zhou explores the revolutionary potential of the genome-edited mouse HUGO-Ab, where endogenous VH and VL genes are replaced by fully human VH and VL genes in situ, enabling the generation of fully human antibody molecules. When combined with microfluidic technology-enhanced single B cell screening, this approach allows for the high-throughput and efficient discovery of antibody drug molecules.
View the On Demand recording
View the webinar slides
 Advancing early-stage bispecific discovery programs towards clinical success, with GS Discovery® and bYlok® technology
Advancing early-stage bispecific discovery programs towards clinical success, with GS Discovery® and bYlok® technology
Jin Lu, PhD, Senior Technical Support Manager, Licensing, Lonza
Broadcast date: June 6, 2024
In this webinar Dr. Jin Lu shows how Lonza’s novel GS Discovery® transient expression platform can boost transient titers by ~30-fold to enable rapid early material supply, and streamline the transition from discovery to stable production. Dr. Lu will also introduce bYlok® bispecific pairing technology – an innovative solution that drives correct heavy-light chain pairing rates in IgG-like bispecifics to >95%. This improves yields of the correct species and streamlines downstream processing, without affecting key performance attributes of the molecule. Dr. Lu will present the latest case studies of bYlok® engineered bispecifics and explore how bYlok® compares to other established pairing solutions.
Key points:
- GS Discovery® supports high titer transient expression to rapidly generate material for early studies
- bYlok® bispecific pairing technology drives correct HC-LC pairing in bispecifics to >95%
- This overcomes issues during downstream processing
- bYlok® and GS Discovery® are accessible via a license agreement
View the On Demand recording
 Harnessing Divergent Species to Access Difficult and Conserved Antibody Targets
Harnessing Divergent Species to Access Difficult and Conserved Antibody Targets
Ross Chambers, PhD, VP of Antibody Discovery, Integral Molecular
Broadcast date: April 25, 2024
To exploit highly conserved and difficult drug targets, including GPCRs and ion channels, monoclonal antibody discovery efforts are increasingly relying on the advantages offered by divergent species such as rabbits, camelids, and chickens. Divergent host species enable robust immune responses against highly conserved binding sites and yield antibodies capable of penetrating functional pockets via long HCDR3 regions. Pan-reactive molecules are often produced by divergent hosts, and these antibodies can be tested in accessible animal models, offering a faster path to clinical development. In this webinar, Dr. Ross Chambers will analyze gaps in therapeutic antibodies that stem from the historic use of mice and examine opportunities to exploit previously inaccessible targets through discovery in alternate species. Examples of preclinical and clinical-stage antibodies raised in divergent species will be highlighted, providing an overview of their success.
View the On Demand recording
View the webinar slides
 Harnessing the Power of Antibody Drug Conjugates: How the Signals™ Research Suite Advances Multi-Modal Drug Development
Harnessing the Power of Antibody Drug Conjugates: How the Signals™ Research Suite Advances Multi-Modal Drug Development
Zev Wisotsky, Ph.D., Sr. Principal Marketing Manager for Biologics in the Signals Research Suite
Broadcast date: March 27, 2024
Antibody-drug conjugates (ADCs) have emerged as an important class of targeted therapeutics, combining the targeting specificity of antibodies with the potency of cytotoxic drugs. Recent advances in ADC research have led to improved stability, efficacy, and safety. There is a clear trend within Antibody therapeutics towards ADCs with the improvement of novel technologies to address a growing cancer population worldwide. However, developing effective ADCs is expensive and poses unique informatics challenges. Researchers must integrate data across multiple modalities – from antibody discovery and engineering to small molecule payload development and optimization. Seamless collaboration between immunologists, protein engineers, chemists, and pharmacologists are essential.
The Signals™ Research Suite is the first integrated informatics platform purpose-built to accelerate multi-modal drug development like ADCs and is composed of a trio of scientific software applications:
- Signals™ Notebook: The premier cloud-based electronic lab notebook that facilitates creation and communication about molecules of interest via hierarchical editing language for macromolecules (HELM), powered Chemdraw®.
- Signals™ VitroVivo: For data analysis, visualization, and curve fitting bioassay results for standardized and consistent results, powered by Spotfire®.
- Signals™ Inventa: Enabling multi-assay or multi-study comparison, powered by Spotfire, with the ability to find the best candidate to move forward, accelerating decision making. Researchers gain better visibility into ADC candidate profiling and can quickly determine structure-activity and structure-property relationships across modalities to select optimal conjugation sites and pairings.
Attendees will gain insights into the latest trends and challenges in ADC development, informed by recent research and advancements in the field. The presentation will also emphasize the importance of integrating various data types and sources in the ADC development process, showcasing how the Signals Research Suite facilitates this integration, thereby driving more efficient, safer drug development.
View the On Demand recording
 3D antibody modeling in Orion
3D antibody modeling in Orion
Jesper Sørensen, head of biomodeling at OpenEye, Cadence Molecular Sciences
Broadcast date: February 15, 2024
OpenEye’s suite of antibody modeling tools, accessed via the cloud platform Orion, includes Specifica’s state-of-the-art antibody sequence analysis tool AbXtract and open-source tools such as ImmuneBuilder for antibody structure prediction. In this webinar, Dr. Jesper Sørensen will guide viewers through the process of modeling antibody structure on Orion, which begins with taking sequences from a selection campaign analyzed using AbXtract, or other methods such as internal or public databases. 3D structures are then generated with the AI-driven structure predictor ImmuneBuilder (requiring only around 100 ms per structure when predicting at the multi-thousand scale). From these structures a wide variety of physico-chemical properties are calculated to enable selection of those antibodies/sequences most likely to be successfully developed into human therapies. Structural diversity in the complementarity-determining region (CDR) can be further explored using knowledge-based loop modeling, while molecular dynamics and enhanced sampling molecular dynamics is used to explore global conformational diversity. Structural families within computed ensembles can be identified by clustering using similarity based on a physically rigorous shape and chemical feature distribution of the CDRs. Individual sequences can be further optimized by single or multi-point mutations and/or loop replacement and these new hypotheses submitted to physico-chemical property profiling and conformational exploration.
View the On Demand recording
 IgE Class Antibody Therapeutics for Cancer
IgE Class Antibody Therapeutics for Cancer
Heather Bax, PhD, King’s College London
Broadcast date: January 23, 2024
Monoclonal IgE antibodies targeting tumor-associated antigens may mediate potent immune-activating functions in tissues, resulting in anti-tumor activity. Dr. Bax will present in vitro and in vivo functions of two anti-cancer IgEs, associations with monocyte/macrophage polarisation, macrophage infiltration, and enriched pro-inflammatory signatures within tumors, the absence of preliminary evidence of type 1 hypersensitivity, and key outcomes of the first-in-class Phase 1 clinical trial; together supporting the potential of IgE-based cancer immunotherapies.
View the On Demand recording
 Strategies for Mitigating the Unpredictability of Fc-Mediated Functions in Antibody Development
Strategies for Mitigating the Unpredictability of Fc-Mediated Functions in Antibody Development
Speaker: Lenny Moise, PhD, VP Research, SeromYx Systems
Broadcast date: November 9, 2023
Optimization of Fc function can lead to the development of safer and more efficacious therapeutic antibody products, but analyses of Fc function may be underexploited in the antibody design process. We introduce a high-throughput platform for antigen-specific testing of Fc effector activities, empowering enhanced hit-to-lead screening in early stages of antibody development for the treatment of cancer, autoimmunity, neurodegeneration, and infectious diseases.
View the On Demand recording
View the webinar slides
 Versatility of Modular Antibodies: From Rapid Format Switch to Fast Screening of Libraries and Bispecifics
Versatility of Modular Antibodies: From Rapid Format Switch to Fast Screening of Libraries and Bispecifics
Speaker: Francisco Ylera, PhD, Bio-Rad
Broadcast date: October 26, 2023
The SpyTag/SpyCatcher protein ligation technology is a versatile method to covalently link two proteins. Addition of the SpyTag protein ligation technology to antibodies creates a modular platform that offers a wide range of new opportunities. In this webinar Dr. Ylera will demonstrate the use of Spytagged antibodies in combination with prefabricated SpyCatcher modules for rapid site-specific labeling, as well as changing antibody valency, species and isotype. Furthermore, Dr. Ylera will present the use of SpyTag for antibody phage display selection (SpyDisplay) and for the generation of prototype bispecific antibodies. The work presented illustrates how these technologies work hand in hand for the generation of new therapeutic lead candidates.
View the On Demand recording
View the webinar slides
 Multiple Innovative Technologies & Platforms Empower Antibody Discovery
Multiple Innovative Technologies & Platforms Empower Antibody Discovery
Speaker: Dr. Long Xu, Director, R&D, Biointron
Broadcast date: September 26, 2023
The development of antibody drugs has greatly depended on the establishment of new technologies and platforms, which are also the key factors that have led to the great success in the development of antibody drugs in the last three decades. Antibodies have a pivotal role in the biopharmaceutical field, but antibody discovery has been constrained by a series of bottlenecks, such as time-consuming and inefficient screening process, poor tissue permeability, difficulties in modular application, and immunogenicity. This talk presents new solutions to these challenges through complete case studies, from antibody discovery to functional assays, antibody optimization, and up to application, using VHH antibody as an example. These case studies will show how new technologies and platforms can be applied to solve common problems in antibody discovery.
View the On Demand recording
View the webinar slides
 Comparing Bispecific Antibody Format Feasibility
Comparing Bispecific Antibody Format Feasibility
Speaker: Ed Horton Ph.D. Chief Commercial Officer
Broadcast date: August 3, 2023
Not every antibody can be combined to produce well-behaved multi-specifics. The valency and geometry of each design can determine the production, target engagement and ultimately the requisite biological functions. In this case study, we selected two established antibody therapeutics, trastuzumab and a humanized OKT3 to produce 17 different bispecific formats to compare the feasibility of each format.
View the On Demand recording
View the webinar slides
Precision high-throughput antibody screening against difficult targets
Speaker: Brandon DeKosky, Assistant Professor in the Department of Chemical Engineering at MIT and a Core Member of the Ragon Institute of MGH, Harvard, and MIT
Broadcast date: January 26, 2023
Mapping the molecular features of antibody immune responses has remained a challenge for many years, partly due to the high genetic diversity that is encoded by millions of single immune cells. This presentation summarizes the past several years of progress we have made in large-scale sequencing and functional screening of antibody immune libraries. We will also share unpublished data and recent advances for antibody discovery in complex cell populations and against difficult disease targets.
View the On Demand recording
 Accelerating Antibody Drug Discovery Through Computational Modeling
Accelerating Antibody Drug Discovery Through Computational Modeling
Speaker: Eliud Oloo, PhD
Broadcast date: October 20, 2022
Abstract: The large size and complexity of biologic molecules creates unique sets of safety, efficacy, and developability hurdles that have to be overcome in order to bring biotherapeutics to market. This webinar will provide an overview of computational modeling strategies for antibody design. The presentation will describe how calculated properties derived from physics-based 3D structural analyses and simulation are applied to not only predict binding affinity, but also identify and mitigate potential liabilities in the development of antibody-based biotherapeutics. Such computational modeling efforts can contribute to significant reductions in project costs and timelines by directing experimental focus toward the most promising candidates.
View the On Demand recording
Best practices in BLI and its applications in screening of a broadly neutralizing antibody of snake venoms
Speakers: Jacob Glanville, PhD, Joel Christian Andrade. PSM, Ben Osborn, PhD.
Broadcast date: August 18, 2022
Biolayer interferometry (BLI) is gaining popularity for protein and small molecule quantitation and kinetics research. The new advancements in biosensors, ease of use, reproducibility and low cost is driving its adoption. Even though BLI is one of the easiest of the tools, as with many other techniques, getting the best data depends on optimization of some key experimental factors. This webinar will discuss the best practices in BLI and result of such implementation by way of an example of screening of a broadly neutralizing antibody of snake venoms.
Snake envenomation results in over 100,000 deaths and 300,000 permanent disabilities in humans annually. Contemporary antivenoms are produced from the polyclonal serum of venom-immunized livestock and are specific to a single or narrow genetic range of related snakes. Could a broadly neutralizing monoclonal antibody, or a cocktail of a few broad components, provide protection from diverse snake venoms? Centi-3FTX-D09, originating from the B-cell memory of a human subject with an extensive history of diverse snake venom exposure, recognized a conserved neutralizing epitope of 3-finger toxins (3FTXs), a dominant snake neurotoxin. Four crystal structures of Centi-3FTX-D09 in complex with 3FTXs from mamba, taipan, krait, and cobra revealed the mechanism of broad neutralization to be epitope mimicry of the interface between these neurotoxins and their native host target, the nicotinic acetylcholine receptor. Centi-3FTX-D09 provided in-vivo protection against diverse 3FTXs and, in combination with the phospholipase inhibitor varespladib, protection against whole venom challenge for diverse, genetically distinct, elapid species.
View the On Demand recording
Understanding memory B cell responses induced by heterologous SARS-CoV-2 exposure
Speaker: Laura M. Walker, PhD
Broadcast date: May 26, 2022
Understanding immune responses following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) breakthrough infection will facilitate the development of next-generation vaccines. Towards this end, Dr. Walker and her colleagues profiled spike (S)-specific B cell responses following Omicron/BA.1 infection in mRNA-vaccinated donors. The acute antibody response was characterized by high levels of somatic hypermutation and a bias toward recognition of ancestral SARS-CoV-2 strains, suggesting the early activation of vaccine-induced memory B cells. BA.1 breakthrough infection induced a shift in B cell immunodominance hierarchy from the S2 subunit toward the receptor binding domain (RBD). A large proportion of RBD-directed neutralizing antibodies isolated from BA.1 breakthrough infection donors displayed convergent sequence features and broadly recognized SARS-CoV-2 variants of concern. Dr. Walker will discuss these findings, which provide insights into the role of pre-existing immunity in shaping the B cell response to heterologous SARS-CoV-2 variant exposure.
View the On Demand recording
 Silence Is Golden: The Importance of Attenuating Effector Functions in Therapeutic Antibodies
Silence Is Golden: The Importance of Attenuating Effector Functions in Therapeutic Antibodies
Speaker: Ian Wilkinson, PhD
Broadcast date: April 28, 2022
Antibodies are nature’s pro-drugs, wonderfully evolved to target pathogens and activate immune systems. For certain indications where ADCC or CDC are required this is ideal, but for many other applications activation of inflammatory responses is unnecessary and potentially highly undesirable. In these situations silenced antibodies with either naturally low effector function or engineered Fc domains are the preferred option. However, many of the commonly used options in the clinic, such as IgG4, LALA or aglycosylation, are widely reported to still have residual Fc receptor binding and cytokine activation in patients.
This presentation will describe the first thorough comparison of most of the generic and proprietary Fc silencing mutations, demonstrating that all previously reported variants show residual binding to Fc receptors. It will also describe the discovery of a novel set of mutations, known as STR, that show no detectable binding to Fcγ receptors and do not elicit inflammatory cytokine responses. Meanwhile, immunogenicity, stability and PK are unaffected. This totally silenced variant has the potential to improve the safety and efficacy of therapeutic antibodies and Fc fusion proteins.
View the On Demand recording
View the webinar slides
Precision Execution of Bispecifics at Scale from Design to Delivery
Speaker: Lisa Prendergast, Ph.D.
Broadcast date: April 7, 2022
Novel therapeutic modalities such as bispecific antibodies are increasingly being explored as more effective alternatives to monoclonal antibodies for a range of diseases. Therapeutics such as bispecifics, can have a combinatorial effect by targeting two antigens, resulting in treatments with enhanced utility, higher efficacy, fewer side effects and less resistance compared to mAbs.
Generating a bispecific antibody, which is correctly and stably paired, is a major production concern. Many solutions require significant changes to native antibody structure, which increases antibody complexity and forces adaptation of downstream processes. While a various platforms have been developed to mitigate Heavy-Light chain (HC-LC) mispairing, there are many other rate limiting steps for efficiently expressing these molecules in a CHO system. bYlok® technology is a design engineering approach that stabilise the interaction between the HC and LC, essentially removing the mispairing problem whilst retaining a more natural antibody structure.
This presentation will introduce you to a mechanistic review of the bispecific pipeline to demonstrate how a various tools and technologies can enable you execute bispecifics. Case studies will be presented to show how the bYlok® technology can be used to stabilise and select for novel bispecifics from a panel of parental immunotherapeutic mAbs. Our data demonstrates that correct heterodimerisation can be achieved consistently and how standard downstream purification processes can be used during production.
View the On Demand recording
Modeling biologic molecules
Speakers: Monica Fernández-Quintero, PhD, and Klaus R. Liedl, PhD, JD
Broadcast date: January 27, 2022
Modeling in Chemistry obviously depends on a strong link to reality. Even though the mathematical description of chemistry has been possible for almost 100 years, realistic modelling has only recently become available due to the massive increase of computing power following Moore’s law. Still, appropriate statistics, initial conditions and boundaries pose considerable challenges. Nowadays, methodological advances and progress in hardware allows the observation of biological systems for relevant time periods. Hence, dynamic processes like reorientations, folding and binding can be seen in atomistic resolution leading to completely new insights.
Describing an antibody’s binding site using only one single static structure limits the understanding and characterization of the antibody’s function and properties, whereas various biophysical properties are governed by its dynamics, e.g., antibody-antigen binding. This limitation is even more pronounced when no experimentally determined structure is available or the crystal structure is distorted by packing effects, which can result in misleading antibody paratope structures. To improve antibody structure prediction and to take the strongly correlated CDR loop and interface movements into account, antibody paratopes should be described as interconverting states in solution with varying probabilities. These kinetically characterized paratope ensembles with their respective state probabilities allow the identification of the dominant conformation in solution, which frequently has been shown to coincide with the binding competent conformation. Therefore, the definition of kinetically and functionally relevant states, so-called paratope states, can be successfully used to improve the accuracy and enhance the predictivity of antibody-antigen docking.
View On Demand recording
View the webinar slides
Innovations from the therapeutics antibody space: obligate bispecific antibodies & T cell engagers
Speaker: Paul W.H.I. Parren, PhD
Broadcast date: October 21, 2021
Landmark advances in the engineering and development of bispecific antibodies bsAbs are enabling unprecedented innovation and versatility in therapeutic antibody concepts. A defining bsAb feature is their potential for novel functionalities — that is, activities that do not exist in mixtures of the parental or reference antibodies. These so-called obligate bsAb are having a tremendous impact and potential in current and future drug development.
Bispecific engagers that target and activate effector cells, most commonly T cells, represent about 50% of bispecific antibodies in development. Here, next generation bispecific T cell engagers (bsTCEs) with a widened therapeutic window characterized by high potency and high tumor selectivity have strong potential for the treatment of both hematological as well as solid cancers. Lava Therapeutics is developing products building on a platform to conditionally and selectively recruit Vγ9Vδ2 T cells for eradicating tumor cells. This γδ T cell subset has been shown to display powerful anti-tumor immune effector activity, to preferentially kill tumor cells relative to normal cells and has a demonstrated ability to infiltrate human tumors. LAVA’s γδ bsTCEs designed to target and engage Vγ9Vδ2-T cells for the development of novel cancer immunotherapies will be discussed.
View On Demand recording
View Dr. Parren’s slides
Antibody Fc Engineering: Designing Antibodies for Cancer, Covid-19, and Beyond
Speaker: Alicia Chenoweth, PhD, Research Associate, St. John’s Institute of Dermatology, King’s College London
Broadcast date: July 8, 2021
Monoclonal antibodies have become one of the most clinical successful therapeutic agents against a range of diseases, including cancer, autoimmune diseases, and most recently SARS-CoV-2. Although engagement of the antigen via the variable Fab portion of the antibody is essential, the function of many therapeutic antibodies also depends, to varying degrees, on the hinge and Fc portion of the antibody and the interaction with receptors on effector cells. Antibody subclass choice is crucial for optimal function and safety of therapeutic antibodies as the functional profile of each subclass differs greatly. Targeted modification of the Fc region and its associated glycan is also a potent and effective approach to tailor the therapeutic function of antibodies for the disease of choice, by improving or reducing the immune cell-associated effector functions and altering the circulating half-life of the antibody. One advantage of these Fc modifications is that they can be easily transferred to antibodies of any target, and so development of novel antibodies against a wide range of diseases have benefited from the modifications previously developed and characterized for different indications.
View On Demand recording
View Dr. Chenoweth’s slides
Staying ahead of the virus: From a multispecies discovery strategy to highly efficacious, clinically suitable multi-antibody cocktails targeting SARS-CoV-2
Speakers: Drs. Debby Kruijsen and Ilse Roodink, ImmunoPrecise Europe
Broadcast date: June 17, 2021
Whereas vaccines significantly contribute to halting transmission of SARS-CoV-2, they are not universally effective. Therapeutic antibodies can fill this gap to efficiently combat SARS-CoV-2. Obviously, long-lasting efficacy of anti-viral approaches heavily depends on the ability to protect against virus variants. As a multi-targeting strategy reduces the risk of mutagenic escape, we have isolated a diversified pool of anti-spike protein antibodies by leveraging our expertise to generate antibody libraries across multiple discovery platforms in different species, to eventually formulate a sensible therapeutic cocktail. A broad range of in vitro characterizations was applied to gain early insight into the epitope landscape and functional characteristics as well as developability profiles. This comprehensive, high-throughput characterization of the obtained lead candidate pool guided the rational combination of high value antibodies into multi-membered cocktails that unlock synergistic effects, significantly boosting neutralization potency in vitro. To accelerate further clinical development of our prioritized antibody cocktails, 2 individual components were subjected to in silico modeling-guided light molecular optimization to minimize liabilities, while in parallel, in vivo efficacy evaluation in a hamster challenge model validated efficient prevention and treatment of SARS-CoV-2 infection following administration of our prioritized antibody cocktails. Although we anticipate that a multi-targeting strategy is the best solution to reduce mutagenic risk escape, we continuously analyze binding of the antibodies of our lead pool for reactivity towards emerging SARS-CoV-2 variants empirically. To date, we screened our prioritized antibody cocktails towards spike protein of the S. African (B.1.351 lineage), Brazilian (P.1 lineage), UK (B.1.1.7 lineage), New York (B.1.526 lineage) and Californian (B.1.429 lineage) strains and confirmed retained binding. Our pandemic preparedness is further strengthened by the readily accessible, diverse pool of antibodies that we generated, which provides enormous possibilities for plug-and-play cocktails to address future SARS-CoV-2 variants.
View On Demand recording
Delivering therapeutic mAbs for COVID-19: What can be done in just one year?
Speaker: Brian Kelley, VIR Biotechnology
Broadcast date: May 20, 2021
Accelerating the timeline from biopharmaceutical discovery to clinical evaluation has long been a focus for industry. For potentially life-saving therapies, the earliest clinical testing enables accelerated pivotal trials and maximum patient benefit. In 2020, novel discovery and development strategies have rapidly evaluated antibodies for passive immunization or treatment of COVID-19, employing CMC timelines from lead identification to clinic cut in half. These strategies combine the latest advances in established platforms with acceptance of higher business risk or costs, while ensuring no increased patient risk. But speed to clinic for COVID-19 antibody therapies must be matched by speed to launch and immediate ramp-up in supply to realize a meaningful impact on the global pandemic. Parallel workflows must be initiated at the onset for process and product development as well as cGMP manufacturing. The initial focus on speed to clinic complements a slower approach to development of the most productive commercial cell line, efficient manufacturing process and optimal drug product configuration. Rapid production of initial clinical material followed by pivotal and commercial production arising from cell line, process and formulation optimization emphasizes the importance of product comparability, structure/function knowledge and appropriate control strategies. Meanwhile, scale-up and technology transfer to large manufacturing facilities starts before the first patient is dosed. These strategies impact the program’s technical and regulatory risk profiles, staff and capital resourcing, and set up trade-offs in multiple areas. This case study describes the development history of a COVID-19 antibody, reviewing CMC milestones from lead identification to preparation of the commercial license application as well as plans for post-licensure opportunities, all in one year. 
View On Demand recording
View Questions and Answers from the webcast
Deep mining of early antibody response in COVID-19 patients yields potent neutralisers and reveals high level of convergence.
Speaker: Dr. John McCafferty, IONTAS
Broadcast date: March 18, 2021
In this webinar, Dr. John McCafferty presents results from the deep-mining of the antibody repertoires of hospitalized COVID-19 patients using a combination of phage display technology and B cell receptor repertoire sequencing to isolate neutralizing antibodies and gain insights into the early antibody response. This comprehensive discovery approach yielded potent neutralizing antibodies with distinct mechanisms of action. In particular, a novel non-ACE2 receptor blocking antibody that is not expected to be affected by any of the major viral variants reported was identified. Potent neutralizing antibodies with near germline sequences within both the IgG and IgM pools at early stages of infection were also found. The study results highlight a highly convergent antibody response with the same sequences occurring both within this patient group and within the responses described in previously published anti-SARS-CoV-2 antibody studies. 
View On Demand recording
View Dr. McCafferty’s slides
Ultra-fast development of an anti-COVID-19 antibody
Speaker: Dr. André Frenzel, YUMAB/CORAT Therapeutics
Broadcast date: February 18, 2021
The SARS-CoV-2 pandemic has spread all over the world in the past year and there is still no efficient treatment available for COVID-19, particularly for patients undergoing severe courses, which often lead to fatal consequences. Antiviral drugs such as virus-neutralizing antibody therapeutics are still urgently needed to save millions of lives. Corat Therapeutics developed a novel SARS-CoV-2 neutralizing, fully human antibody derived from convalescent patients. Ultra-fast development strategies shortened timelines from discovery to GMP manufactured material to less than 8 months. This was possible by parallelizing discovery and development steps, as well as performing crucial steps on risk. First-in-human studies were recently initiated. This webinar gives an overview of the challenges faced during discovery and development of the lead antibody, COR-101. 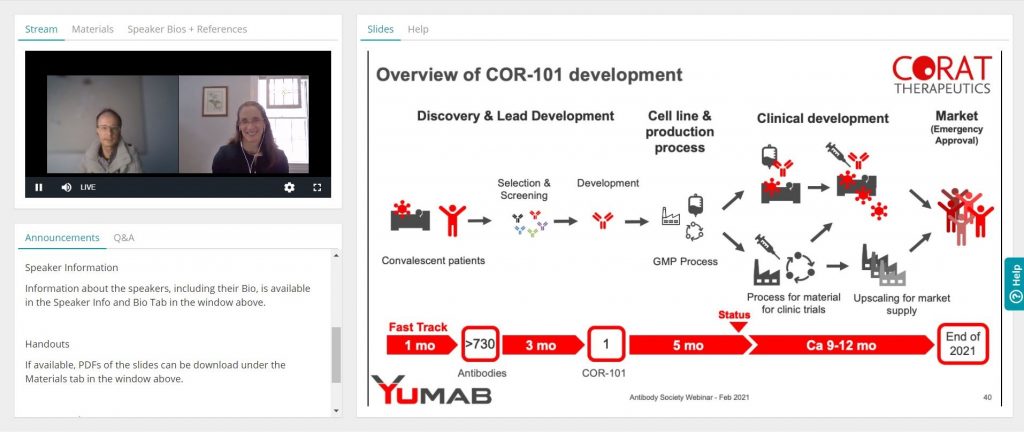
View On Demand recording
Antibody Discovery in the Cloud: Using NGS to expand the universe of selectable antibodies
Speakers: Drs. Andrew Bradbury and M. Frank Erasmus, Specifica
Broadcast date: January 21, 2021
The Specifica Generation 3 platform is able to generate 500-5000 different antibody clonotypes against targets of interest, with over 80% of selected antibodies having no measurable biophysical liabilities and 20% having subnanomolar affinities. The most common approach to selecting antibodies from display technologies involves low-throughput random colony screening. However, this misses many potential therapeutic leads, particularly when diversity is high. Specifica uses next generation sequencing (NGS) to build its libraries as well as characterize selection outputs. In order to fully exploit the universe of selectable antibodies, Specifica has developed a cloud-based software platform, designed exclusively for antibody engineers and bioinformaticians, to enable a streamlined identification of leads with broad epitope coverage. Application of this to selection outputs has increased the number of clonotype leads by five to ten fold over random colony screening, significantly expanding the explorable paratope space. 
View On Demand Recording
All Antibody Society webinars are open access, and can be viewed for free.
Note: The webinar platform and registration process is managed by Community Brands.
Their privacy policy is here.